A New Cancer Treatment Therapy
Parsa Shahbodaghi*
Cancer is defined as a disease that results from a cell in one’s body dividing abnormally. There are multiple events that could result in a cell becoming cancerous. The first is that genes that normally promote cell division or proto-oncogenes mutate, meaning that their DNA sequences change so that these proto-oncogenes become cancerous oncogenes. The oncogenes then cause the cell to divide uncontrollably to form a tumor that could then spread to the rest of the body. Cancer can also arise from mutations in genes that are supposed to stop the cell from dividing, also known as tumor suppressor genes. However, mutations in both proto-oncogenes and tumor suppressor genes are required for a cell to become cancerous. [1]
There are multiple genes that can become mutated and cause cancer. This means that there are multiple cancerous pathways, each having its own defining features. Therefore, scientists need to develop treatments that are specifically targeted to a particular type of cancer.
Before the advent of genomics, the best-known way in which doctors were to treat cancer was to expose a patient to toxic treatments, involving radiation and chemotherapy with the intent of killing cancerous cells. This can be considered as a brute-force approach of treating cancer, because the toxins not only target cancer cells but also target the healthy cells that people need in order to function. [2] That’s why individuals undergoing these treatments can become lethargic to the point where they are unable to function normally. Chemotherapy and related treatments are relatively ineffective in treating neuroblastoma, leukemia, and pancreatic cancer because destroying cancer cells in these areas would also kill cells that are necessary for the patient’s survival. Therefore, it is highly unlikely that a patient would recover from these ailments and these types of cancer usually result in one’s death.
New drugs typically focus on preventing proteins that are involved in the cell-growth pathway from interacting with each other. [3] They therefore stop the process of cell division and hinder the tumor’s ability to grow further. Patients have varying responses to these drugs so this type of therapy can only be effective under certain circumstances.
A new treatment method is known as Kanzius RF therapy. [2] It involves using golden or carbon-based nanoparticles, radio waves, and antibodies to kill cancer. [4] Scientists can target these golden or carbon-based nanoparticles into a cell by means of using an antibody that can recognize a particular type of cancer cell. The nanoparticles move inside the tumor and, when heated to extremely high temperatures by means of radio waves, race around, destroying the cellular structures of the cancerous cells. [4] Scientists researching this method reported experimental results, showing that every cancer cell that was injected with the nanoparticles was killed when exposed to radio waves. [5]
Since scientists can develop antibodies to target cancer cells, the therapy could therapeutically be used against any type of cancer. The therapy does not merely hinder cell division with the possibility of curing disease, like the drugs currently available on the market; it kills all cells that have the nanoparticles within them. It is important to note that this therapy is not ready for use in patients. It has shown promise in the lab, where it has been observed to kill all cancer cells, but it has just recently entered clinical trials. [2] The therapy is currently being used on large animals. [6]
* Parsa Shahbodaghi is an Asssitant Editor for TuftScope. This is a special web only post on biomedical advances accompanying the Spring 2010 issue.
References
1. Campbell N., Reece J., Urry L., Cain M., Wasserman S., Minorsky P., Jackson R. "Biology" p. 374-377 2008. San Francisco: Pearson Benjamin Cummings
2. Laloup, Jennifer. "Cancer Therapy Without Side Effects Nearing Trials". Wired. 03/03/2010. [Link]
3. Harmon, Amy. "A Drug Trial Cycle: Recovery, Relapse, Reinvention". The New York Times. 03/02/2010. [Link]
4. Stahl, Leslie. "Homemade Cancer Machine Shows Promise". CBS News. 03/02/2010.[Link]
5. Gannon C. J., Cherukuri P., Yakobson B. I. , Cognet L., Kanzius J.S., Kittrell C. , R. Weisman B, Pasquali M. , Schmidt H. K. , Smalley R. E. , Curley S. A. "Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field.” 2007. Cancer 110(12): 2654-65.
6. Kanzius Cancer Research. [Link]
Showing posts with label Biomedicine. Show all posts
Showing posts with label Biomedicine. Show all posts
Monday, May 3, 2010
Thursday, March 25, 2010
Down syndrome abnormalities may actually be due to gene underexpression, not overexpression

Down syndrome is a chromosomal abnormality that affects human chromosome 21, and occurs in about 1 in 800 live births. The most common type is known as Trisomy 21, which results from a nondisjunction event during meiosis. Meiosis occurs in such a way that the affected gamete receives two copies of chromosome 21 instead of just the usual one copy. When fertilization occurs with another normal gamete, the resulting zygote then contains three copies of the chromosome - hence the name Trisomy 21. Other common types of Down syndrome include Mosaicism, where nondisjunction occurs after fertilization, so that only some cells in the body contain the extra 21st chromosome. Robertsonian translocation is another, and it occurs when one of the parents of the affected individual has part of their chromosome 21 attached to chromosome 14 or 15. This often results in an uneven distribution of chromosome 21 in their gametes.
Why chromosome 21? Because it is the smallest of all the human chromosomes, it is believed that it is more likely to become 'lost' in the process of meiotic division. In addition, the risk of developing Down syndrome spikes if the individual's mother is over the age of 40, as the likelihood of error during meiosis increases with age.
Common physical characteristics of DS-affected individuals include a round face, flattened profile, almond shaped eyes, and short stature. They may also have reduced intellectual capacity, though this varies greatly from person to person. Many are at risk for developing congenital heart defects. It may seem like common sense to assume that defects like these are a product of gene overexpression - there is an entire extra chromosome at work here. In the study cited below, scientists have found that microRNA-155, located on chromosome 21, is in fact overexpressed in heart and brain cells; but its overexpression leads to the underexpression of the transcription factor MeCP2, which controls the expression of several genes. Their study with mice has shown that the amount of protein can be raised to a normal level by treatment with a drug called antagomir.
Reference: Ohio State University. "New Theory of Down Syndrome Cause May Lead to New Therapies." ScienceDaily 24 March 2010. 25 March 2010 <http://www.sciencedaily.com /releases/2010/03/100323121839.htm>.
Photo credit: Human male karyotype showing three copies of chromosome 21. University of New South Wales Embyology. Can be found here.
Linda Le is a contributing writer on biomedical research to TuftScope for Spring 2010.
Labels:
Biomedicine

Monday, March 8, 2010
'Death messenger' molecule CD95 marks cells for apoptosis

Apoptosis, or programmed cell death, is actually a critical bodily process despite its seemingly formidable name. It is a sort of ‘cell suicide’, in which certain morphological changes occur within the cell and result in its destruction. It functions as a mechanism by which cells can fight off infection and starvation; if one cell is damaged or in danger, it sacrifices itself for the greater good of the organism by getting rid of itself through apoptosis. In addition, programmed cell death is an integral part of proper tissue development. During embryonic development, humans grow webbed fingers and toes; if it weren’t for apoptosis, we would retain those webs after birth, which is a condition known as syndactyly. Because cell death is in large part about keeping damage from spreading, it is not hard to see that apoptosis also aids in tumor suppression and cancer prevention.
One of the players in the apoptotic pathway is a receptor protein called CD95. When another protein, CD95L, binds to this receptor, a death inducing signaling complex (DISC) is formed. This, in turn, sets off a signaling cascade that eventually leads to cell apoptosis. While this may be beneficial in the case of fighting off invading pathogens and the like, what happens when the ‘death messenger’ response overshoots and ends up killing off cells that could otherwise be healed? Researchers explored this in the case of spinal cord injuries in mice. They found that CD95L sets off an inflammatory response that recruits macrophages to the site of injury and marks the injured cells for apoptosis. When CD95L was switched off, however, the mice were allowed to heal. Such a discovery could lead to more informed approaches to inflammatory and immune-related diseases.
Reference: Helmholtz Association of German Research Centres. "'Death Messenger' Molecule Causes Inflammation After Spinal Cord Injury, Prevents Healing." ScienceDaily 6 March 2010. 8 March 2010
Photo credit: Reproductive and Cardiovascular Disease Research Group. Can be found here.
Linda Le is a contributing writer on biomedical research to TuftScope for Spring 2010.
Labels:
Biomedicine

Friday, February 26, 2010
PPAR-g signaling protein is defective in cystic fibrosis patients
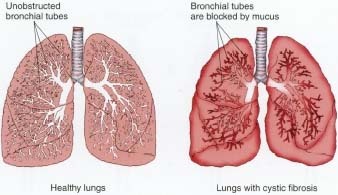
Cystic fibrosis is a life-shortening, autosomal recessive disease that affects around 70,000 people worldwide, and is seen predominantly in Caucasians. It is marked by the production of a thick, sticky mucus in both the lungs and pancreas due to a defect in the gene CFTR. While most affected individuals die in their 20s and 30s, medical advances have led to a longer life expectancy of around 37 years.
CFTR codes for a transmembrane regulator protein, and is responsible for keeping the water and salt concentrations of cells in check. A defect in the protein, then, causes mucus buildup in the lungs, which then leads to various problems such as difficulty breathing, excessive coughing, inflammation of lung tissue, and improper blood and oxygen circulation. The pancreas, an organ partly responsible for digestion, also produces thick secretions that disrupt digestive enzyme activity and decrease nutrient intake to the body. Due to the trouble in breaking down and absorbing certain foods, gastrointestinal problems are common. Because the pancreas is also responsible for producing insulin, it is not unusual for cystic fibrosis patients to develop diabetes. Medical treatments for the disease include anti-inflammatory drugs, mucus-thinners, and individual techniques for clearing out airway passages.
In a new study at UC San Diego, researchers have found that a defect in a protein called PPAR-g is found in the cells of both cystic fibrosis patients and mice inflicted with the disease. Unsurprisingly, the protein is involved with the regulation of ions across cell membranes. The researchers found that a drug called rosiglitazone, when applied as a treatment in mice, serves to normalize PPAR-g gene expression and therefore mitigates the disease.
Reference: University of California - San Diego (2010, February 15). Defective signaling pathway sheds light on cystic fibrosis. ScienceDaily. Retrieved February 26, 2010, from http://www.sciencedaily.com /releases/2010/02/100214143133.htm
Photo credit: Depiction of healthy lungs on left; lungs with cystic fibrosis on right. Encyclopedia of Human Diseases and conditions. Can be found here.
Labels:
Biomedicine

Wednesday, February 17, 2010
Immune system aids in kidney tissue regeneration
 When lizards are attacked, they have the remarkable ability to detach their tails from their body in order to confuse their enemy, and then, to grow it back. If a salamander gets its leg chopped off, it can regenerate another one in a matter of weeks. So why can’t humans?
When lizards are attacked, they have the remarkable ability to detach their tails from their body in order to confuse their enemy, and then, to grow it back. If a salamander gets its leg chopped off, it can regenerate another one in a matter of weeks. So why can’t humans? From an evolutionary standpoint, it would be energetically wasteful to regenerate a body part that is not necessary for survival, like a finger. Conversely, if an important organ like the heart were significantly damaged, the individual would likely not survive long enough for regeneration to actually take effect. Instead, much of our bodies’ effort seems to have gone into wound healing, which actually inhibits potential cell regeneration. In 1974, Dr. Cynthia Illingworth found that young children could regenerate fingertips, but only if their wounds were left open rather than surgically re-covered with skin. In addition, studies in mice have shown that the formation of scar tissue blocks cell signaling necessary for regeneration. When salamanders lose their limbs, instead of covering the wound, cells near the site of the injury de-differentiate and form a group of cells collectively known as a blastema. They can then re-differentiate and build the lost structure, but the cells do not exhibit full pluripotency; instead, old skin cells become skin cells and former bone cells become bone once again. The inability of many higher-order species to undergo this process may be merely one explanation as to why regeneration in those species is rare.
Though humans do not have a great capacity for regeneration, the liver is able grow back from around 25% of its tissue. Research has suggested that this is achieved through the proliferation of cell division, rather than reversion back to an embryonic state. A recent study has discovered a mechanism for the regeneration of damaged tissue in the kidneys; interestingly enough, it occurs via the immune system.
Reference: Cincinnati Children's Hospital Medical Center. "Scientists Discover Molecular Pathway for Organ Tissue Regeneration and Repair." ScienceDaily 16 February 2010. 17 February 2010 <http://www.sciencedaily.com /releases/2010/02/100215174134.htm>.
Photo credit: Scientific American. Can be found here.
Linda Le is a contributing writer on biomedical research to TuftScope for Spring 2010.
Labels:
Biomedicine

Sunday, February 7, 2010
Spread of viruses occurs faster than originally thought

When it comes to viruses, the definition of ‘living’ and ‘non-living’ becomes a complicated issue. Some would argue that merely having a protein coat and genetic material does not qualify a living thing. However, when we look at virus' structural variety and incredible ability to replicate in a wide range of hosts, it is undeniable that they are indeed responding to their environment and evolving alongside other life forms.
Viruses have viral receptor proteins on their surface, which recognize specific host cells. They then insert their genetic material into the cell, and either create additional copies of their genome on their own (in the case of RNA viruses), or rely on the host cell’s machinery (DNA viruses) for the same purpose. Of course, the host cell has its own ways of recognizing these foreign invaders. A method called RNA interference cuts off the production of viral mRNA, hindering the reproductive cycle. In humans, killer T-cells recognize viral fragments on the surface of an infected cell and mark it for apoptosis. Interestingly, the HIV virus undergoes rapid mutations that change the amino acid sequence on its viral coat, enabling it to escape both vaccines and the killer T-cell response.
Researchers at Imperial College London have recently captured a new video of the vaccinia virus spreading throughout cells over the course of 16 hours. The virus appears to spread at a faster rate than its replication cycle allows; it apparently has evolved a mechanism which allows it to recognize which host cells have and have not yet been infected, thereby saving time and effort. This new information could change the way we approach viral propagation, and hopefully, it will lead to more advanced medical strategies in treating viral diseases.
Reference: Video of virus in action shows viruses can spread faster than thought possible
Image: Electron micrograph of a vaccinia virus. Can be found here.
Linda Le is a contributing writer on biomedical research to TuftScope for Spring 2010.
Labels:
Biomedicine

Tuesday, February 2, 2010
Prions play a role in both healthy and infected cells

Until Stanley B. Prusiner’s discovery of prions in 1982, it was commonly believed that infections occurred via the transmission of nucleic acids, be it through bacterial or viral particles. Prions, which are now known to cause several neurodegenerative diseases, such as Creutzfeldt-Jakob and mad cow disease, are made up solely of proteins – and are capable of spreading without any DNA or RNA intermediate.
Certain post-translational modifications result in the transformation of normal prion proteins (PrP), which are found in healthy neurons, into abnormal, infectious proteins (PrPSc). These modifications change the proteins’ secondary structure, conferring greater stability and rigidity. Diseased proteins are then capable of influencing the structure of other nearby PrP and converting them into PrPSc mutants. This process may be spontaneous, inherited (through a mutation in the gene encoding the normal protein), or may occur through infectious means (i.e. transplantation). Once the transformation into PrPSc has taken place, the infected prions aggregate inside cells, disrupt cell function, and eventually lead to death.
Prions are resistant to proteases, which break down proteins. They do not act as recognizable antigens, and may therefore go undetected for long periods of time. Many of the diseases they cause (otherwise known as transmissible spongiform encephalopathies) may also lay latent for years, and they are all fatal in humans. Needless to say, our understanding of prions has a long way to go.
The article below discusses studies conducted by Swiss researchers, who are not concerned with the mutant PrPSc, but rather with their healthy predecessor, PrP. Because prions are a relatively new concept in the pathogenic world, these researchers believe that we must first understand their normal function before we can delve into disease.
Reference: Prusiner, Stanley B. “Prions”. PNAS November 10, 1998 vol. 95 no. 23 13363-13383. See also: Prions 'may keep nerves healthy'.
Image credit: Normal prion on left, diseased prion on right. Can be found here.
Linda Le is a contributing writer on biomedical research to TuftScope for Spring 2010.
Labels:
Biomedicine

Saturday, January 30, 2010
Sonic hedgehog signaling pathway influences response to cancer therapy
A crucial component of early vertebrate development is Sonic Hedgehog (Shh), a transcription-regulation protein. It is named after its mutant phenotype found in Drosophila, which manifests itself in pointy denticles that cover the embryo, as well as the popular video game character. Shh is a morphogen that directs proper limb patterning, as well as the development of certain organs such as the spinal cord and heart.
The discovery of Shh was a major stepping-stone in the field of evolutionary developmental biology, a field that is concerned with how developmental processes on an embryonic level are tied into the big picture of evolution. Shh provided part of the answer to the question of how an entity as small as the embryo could inherently ‘know’ its orientation and consequently perform the incredible task of positioning the development of its body parts. It turns out that Shh plays an active part in the Zone of Polarizing Activity (ZPA), which determines the orientation of a developing limb bud. Shh forms a concentration gradient as it diffuses from the ZPA, and the levels of the morphogen (as well as several other components at play) determine digit formation. In short, it is why you have all five fingers, all in the correct spot.
Shh mutations in humans may cause holoprosencephaly, which is characterized by the failure of the brain to undergo complete division of the left and right hemispheres, cleft lip, and in severe cases, cyclopia. While studying this condition, researchers found that the chemical cyclopamine (named after the one-eyed sheep that had resulted from cyclopamine exposure) is an inhibitor of the Shh signaling pathway. Because it restricts growth, cyclopamine has been utilized as a tumor suppressor in several different cancers.
The article below highlights a very recent finding by scientists at the University of Texas M. D. Anderson Cancer Center, which sheds light on Sonic hedgehog gene variation, and how it has impacted our knowledge of medicine: in this case, cancer therapy.
Linda Le is a contributing writer on biomedical research to TuftScope for Spring 2010.
The discovery of Shh was a major stepping-stone in the field of evolutionary developmental biology, a field that is concerned with how developmental processes on an embryonic level are tied into the big picture of evolution. Shh provided part of the answer to the question of how an entity as small as the embryo could inherently ‘know’ its orientation and consequently perform the incredible task of positioning the development of its body parts. It turns out that Shh plays an active part in the Zone of Polarizing Activity (ZPA), which determines the orientation of a developing limb bud. Shh forms a concentration gradient as it diffuses from the ZPA, and the levels of the morphogen (as well as several other components at play) determine digit formation. In short, it is why you have all five fingers, all in the correct spot.
Shh mutations in humans may cause holoprosencephaly, which is characterized by the failure of the brain to undergo complete division of the left and right hemispheres, cleft lip, and in severe cases, cyclopia. While studying this condition, researchers found that the chemical cyclopamine (named after the one-eyed sheep that had resulted from cyclopamine exposure) is an inhibitor of the Shh signaling pathway. Because it restricts growth, cyclopamine has been utilized as a tumor suppressor in several different cancers.
The article below highlights a very recent finding by scientists at the University of Texas M. D. Anderson Cancer Center, which sheds light on Sonic hedgehog gene variation, and how it has impacted our knowledge of medicine: in this case, cancer therapy.
Reference: University of Texas M. D. Anderson Cancer Center (2009, December 10). Sonic Hedgehog variations linked to recurrence, survival and response to therapy of bladder cancer. ScienceDaily. Retrieved January 30, 2010, from http://www.sciencedaily.com /releases/2009/12/091209114144.htm.
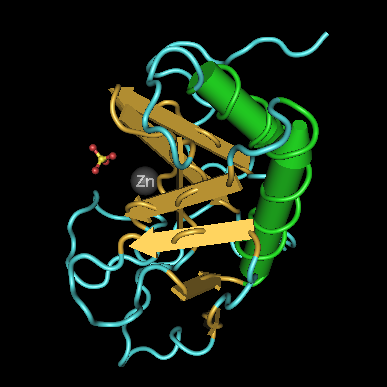
Image Credit: 3D structure of the signaling domain of the murine hedgehog protein. Wikipedia Commons. Available here.
Image Credit: 3D structure of the signaling domain of the murine hedgehog protein. Wikipedia Commons. Available here.
Linda Le is a contributing writer on biomedical research to TuftScope for Spring 2010.
Labels:
Biomedicine

Subscribe to:
Posts (Atom)